This common arrangement of the periodic table separates the lanthanides (lanthanoids) and actinides (actinoids) (the f-block) from other elements. The wide periodic table incorporates the f-block. The extended periodic table adds the 8th and 9th periods, incorporating the f-block and adding the theoretical g-block.

Periodic Table showing Groups

Periodic Table showing Periods
Although there were precursors, the current presentation's invention is generally credited to Russian chemist Dmitri Mendeleev, who developed a version of the now-familiar tabular presentation in 1869 to illustrate recurring ("periodic") trends in the properties of the then-known elements. The layout of the table has been refined and extended over time, as new elements have been discovered, and new theoretical models have been developed to explain chemical behavior.

The modern periodic table is
Since the periodic table accurately predicts the abilities of various elements to combine into chemical compounds, use of the periodic table is now ubiquitous within the academic discipline of chemistry, providing a useful framework to classify, systematize, and compare many of the many different forms of chemical behavior. The table has found many applications not only in chemistry and physics, but also in such diverse fields as geology, biology, materials science, engineering, agriculture, medicine, nutrition, environmental health, and astronomy. Its principles are especially important in chemical engineering.

The Periodic Table
One of the strengths of Mendeleev's presentation is that the original version accurately predicted some of the properties of then-undiscovered elements expected to fill gaps in his arrangement. For example: "eka-aluminium", expected to have properties intermediate between aluminium and indium, was discovered with said properties in 1875 and named gallium. No gaps remain in the current 118-element periodic table; all elements from hydrogen to plutonium except technetium, promethium and neptunium exist in the Earth in macroscopic or recurrently produced trace quantities. The three said exceptions do exist naturally, but only in trace amounts as the result of rare nuclear processes from decay of heavy elements. Every element through Copernicium, element 112, has been isolated, characterized, and named, and elements 113 through 118 have been synthesized in laboratories around the world.

the modern periodic table

Modern Periodic Table
While plutonium is now included among the 91 regularly occurring natural elements, and technetium, promethium, and neptunium also occur naturally in transient trace amounts, these four elements were first identified and characterized from technologically produced samples. Numerous synthetic radionuclides of various naturally occurring elements have been produced as well.
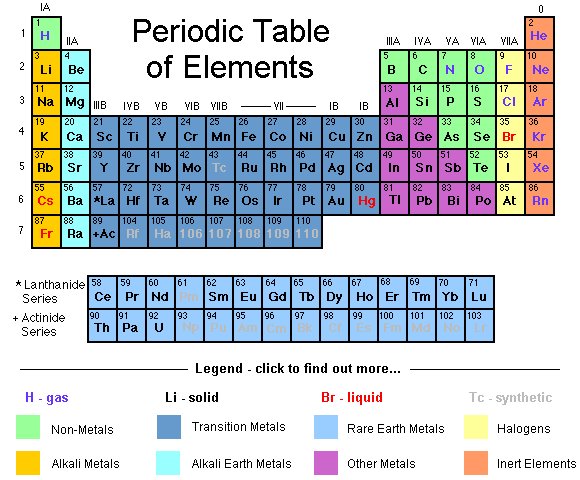
Groups (Periodic Table):
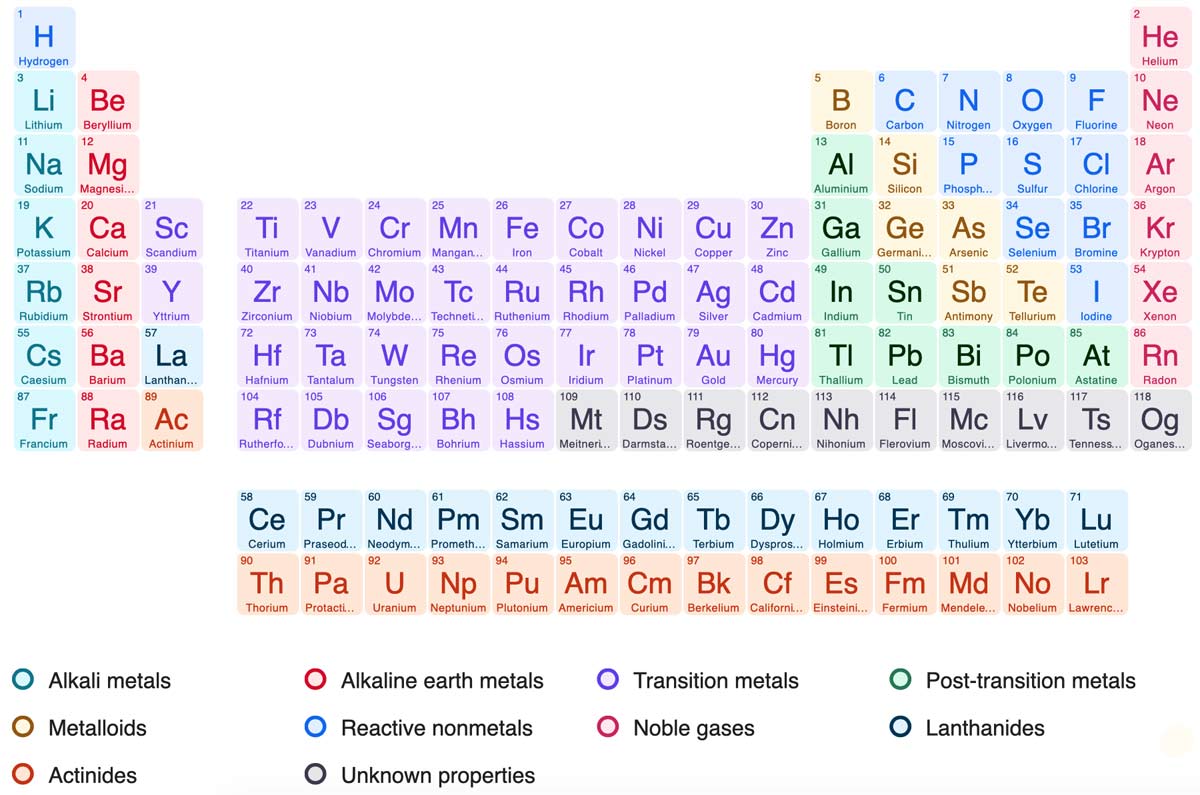
periodic table of elements

the real periodic table.
Production of additional synthetic elements beyond atomic number 118 is being pursued; whether the next elements will neatly fill an eighth period or require modifications to the overall patterns of the present periodic table remains unknown.

Groups are the
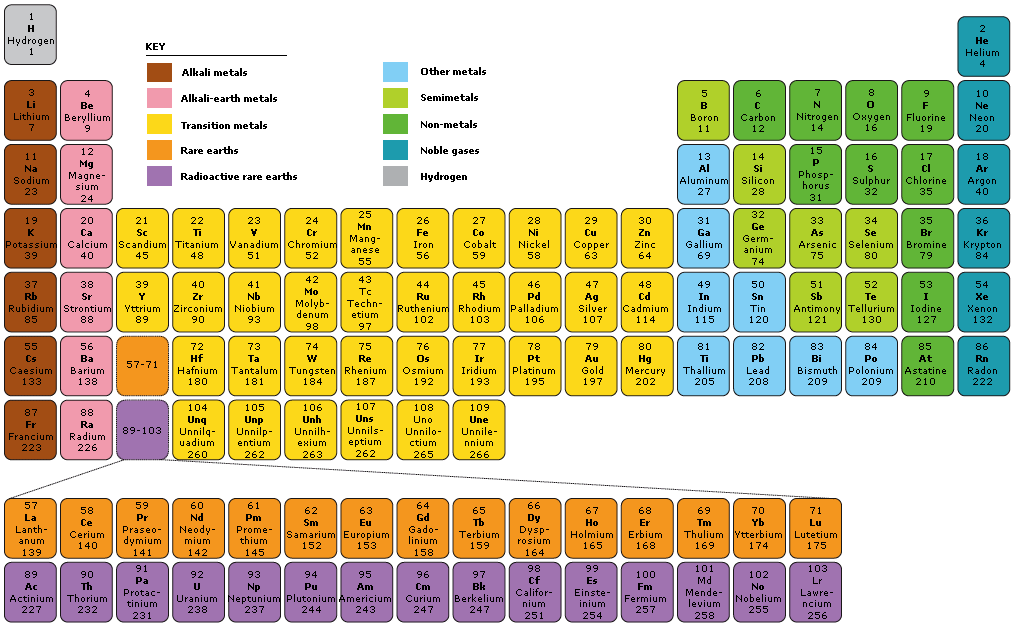
a table that groups,
The main value of the periodic table is the ability to predict the chemical properties of an element based on its location on the table. It should be noted that the properties vary differently when moving vertically along the columns of the table than when moving horizontally along the rows.

The periodic table can be

The Periodic Table of the

periodic table

Periodic Table

PeriodicTable from

Periodic Table showing Groups

Periodic Table showing Periods
Although there were precursors, the current presentation's invention is generally credited to Russian chemist Dmitri Mendeleev, who developed a version of the now-familiar tabular presentation in 1869 to illustrate recurring ("periodic") trends in the properties of the then-known elements. The layout of the table has been refined and extended over time, as new elements have been discovered, and new theoretical models have been developed to explain chemical behavior.

The modern periodic table is
Since the periodic table accurately predicts the abilities of various elements to combine into chemical compounds, use of the periodic table is now ubiquitous within the academic discipline of chemistry, providing a useful framework to classify, systematize, and compare many of the many different forms of chemical behavior. The table has found many applications not only in chemistry and physics, but also in such diverse fields as geology, biology, materials science, engineering, agriculture, medicine, nutrition, environmental health, and astronomy. Its principles are especially important in chemical engineering.

The Periodic Table
One of the strengths of Mendeleev's presentation is that the original version accurately predicted some of the properties of then-undiscovered elements expected to fill gaps in his arrangement. For example: "eka-aluminium", expected to have properties intermediate between aluminium and indium, was discovered with said properties in 1875 and named gallium. No gaps remain in the current 118-element periodic table; all elements from hydrogen to plutonium except technetium, promethium and neptunium exist in the Earth in macroscopic or recurrently produced trace quantities. The three said exceptions do exist naturally, but only in trace amounts as the result of rare nuclear processes from decay of heavy elements. Every element through Copernicium, element 112, has been isolated, characterized, and named, and elements 113 through 118 have been synthesized in laboratories around the world.

the modern periodic table

Modern Periodic Table
While plutonium is now included among the 91 regularly occurring natural elements, and technetium, promethium, and neptunium also occur naturally in transient trace amounts, these four elements were first identified and characterized from technologically produced samples. Numerous synthetic radionuclides of various naturally occurring elements have been produced as well.

Groups (Periodic Table):

periodic table of elements

the real periodic table.
Production of additional synthetic elements beyond atomic number 118 is being pursued; whether the next elements will neatly fill an eighth period or require modifications to the overall patterns of the present periodic table remains unknown.

Groups are the

a table that groups,
The main value of the periodic table is the ability to predict the chemical properties of an element based on its location on the table. It should be noted that the properties vary differently when moving vertically along the columns of the table than when moving horizontally along the rows.

The periodic table can be

The Periodic Table of the

periodic table

Periodic Table

PeriodicTable from
No comments:
Post a Comment